About Amalgamation and Using Mercury

.

About Amalgamation and Using Mercury
|
 |
||
|
. |
Interested in using mercury to recover gold and silver and get all the gold out of your black sands? There is often some very small sized gold in our concentrates, and it can be a lot of work to get all the gold out. Yet we make a lot of effort to capture the gold and we don't want to throw any fine gold away as it can be so valuable. Before you get going too far, take a look at the following information about getting all the gold out of your black sands and producing a clean, saleable placer gold product. Take a look at amalgamation....... |
 |
|
| Amalgamation has been used for thousands of years to recover small sized or fine gold. Historically, it was used in many operations to increase gold recovery. In spite of this I recommend against using it in almost all applications. Especially for the small prospector, there are many better options available for separating your fine gold from black sand. Here is a link to my page on Processing Black Sands. Many prospecting shops no longer even sell mercury. In spite of its potential danger, I would like to present some information about using Mercury recover fine gold because of its historical significance and general interest to the individual prospector and miner. If you decide to use Mercury with your prospecting operations, it is mandatory that you take care to learn all the necessary safety precautions and use them in every step of your work. | ||
| Basically, amalgamation is the practice of bringing free gold
particles into contact with mercury. When clean gold comes into contact with mercury, the
two substances mix to form a compound called amalgam – an amalgam is simply an alloy
of gold and mercury. The gold literally is dissolved into the mercury. This allows the
collection of very small sized particles of gold. At the end of the operation the mercury
and gold amalgam are collected after which the two are separated, and the mercury
recycled. Now larger gold and nuggets will not be completely converted (except over a very
long time) and only a thin coating of amalgam forms. However, large nuggets should not be
captured with mercury, it should be saved to use in recovering fine sized gold. Mercury and free gold can be brought together in a number of ways, but to mix well, both the mercury and gold must be clean. Coatings, especially those which are oily in nature, prevent the two from mixing. In the old days, miners placed mercury in the riffles of sluices, dry washers, and similar devices to aid capture of fine gold. At the base of most of the old stamp mills was a plate amalgamator – a device with a metal plate coated with a thin film of mercury on the surface. Crushed ore or concentrates was fed slowly over the plate, and gold adhered to the mercury. Using “open system” methods like these where any mercury lost through the system went out with the tailings into the environment would be crazy today, and simply an invitation for a big dollar citation from every environmental agency that has jurisdiction over you. |
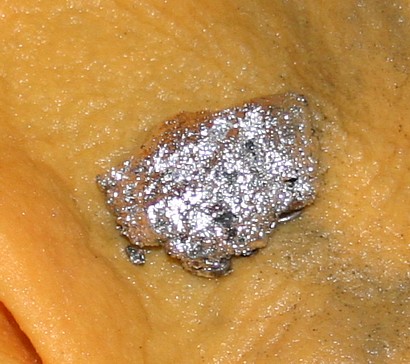 Gold-Mercury Amalgam |
|
| More reasonable (and certainly safer to your wallet) are closed systems where the tailings do not just flow out into the environment. Barrel amalgamators are rotating containers, some of which contain steel rods or balls for grinding. This grinding action helps clean the gold to ensure good contact with the mercury. These barrels are made to rotate slowly for maximum contact, mixing the feed with the mercury, and also to prevent flowering of the mercury. Flowering is a situation where Mercury breaks up into the thousands of tiny pieces. These tiny particles of Mercury are difficult to recover and also increase your loss of fine gold. Occasionally, the amalgamation process does not collect as much gold as anticipated. This type of result usually occurs when the formation of amalgam is inhibited due to poor contact between the gold and the mercury. This happens most commonly when the gold is very fine or when it is tarnished by a surface or film. Also, the feed material may be contaminated with any grease, oil, or any other inhibiting agent. | ||
The greatest potential disadvantage of amalgamation is the health hazard presented by mercury. Mercury is toxic. It attacks your nervous system doing damage over a long period time. It has the characteristic of not being easily excreted from the body, so if you feel fine, it still may affect you in the long term. If you ingest a large amount of Mercury for example, it will stay in your body for a long time. On the other hand, if you ingest only a little Mercury each day it will accumulate in your body until, if you take no action, it can eventually produce toxic symptoms such as hair loss etc. The term “Mad Hatter”, or “mad as a Hatter” derives from the very real situation that hat makers in the 18th century used Mercury to process felt, a commonly used hat making material of that time. After years of being in the business, exposure to small amounts of mercury vapor each day quite literally made them insane. It's certainly not the type of situation which you would like to also inflict upon yourself. Mercury has a relatively high vapor pressure, which means that at
normal temperatures if you left a bowl of mercury out in the air a significant amount
would vaporize and would evaporate to the air. If you continued to breathe this Mercury
containing air you certainly would ingest a significant amount of Mercury. The most
dangerous thing about Mercury is that the lungs will readily absorb the vapors. While
Mercury is only slowly absorbed through the skin is still worthwhile to protect yourself.
Even though Mercury was once used in various forms for medical purposes, most of these
uses have now been banned for health reasons. |
 |
|
There are a few common sense
precautions which you must follow when working with Mercury. These include: Ø When working with Mercury always use latex gloves, they are cheap and provide good protection. Ø Always store Mercury in tightly closed containers (unbreakable is better, and not ones made of Aluminum). Ø Always put a layer of water on top of the Mercury unless it is charged Mercury. The water suppresses the mercury vapor. Ø Never heat Mercury or amalgam indoors or in any enclosed space. If you must heat Mercury do it in the outdoors or a well-ventilated area. Ø Always keep yourself upwind from hot mercury. Do not breathe the fumes. Stand upwind of cold Mercury as well when it is practical. Ø Avoid spilling mercury. It splatters into tiny bits and is very difficult to clean up. Prevention of the problem is the best way to deal with the situation. Ø Never try to distill (retort) Mercury in a glass retort, as it will
shatter. Mercury retorts are made of strudy iron. |
||
"Clean" Mercury can mean different things to different
people. It most commonly means that it is clean enough that clean gold touching it will be
amalgamated. There are a number of ways to clean Mercury, perhaps the easiest is to
squeeze it through a wet chamois. Clean Mercury will pass through the chamois and come out
as tiny droplets. This process will filter
out any junk and most oils that contaminated the Mercury, and the process is fairly quick.
Unless your Mercury was extremely oily, it should now be clean enough to use in
amalgamation. A recent innovation in the use of mercury in treating concentrates is to charge it by introducing a small amount of a very active metal like sodium or potassium. The resulting product is called "charged mercury" and will amalgamate with gold in an aggressive manner. In many situations where the gold might not amalgamate because it was just slightly coated, the charged Mercury will attack it through the coating and successfully amalgamate. "Charged mercury" is nothing magical it is really not that difficult to make. You could just take some sodium metal and drop little chunks of it into liquid mercury, but that is dangerous and sodium metal is also dangerous and hard to acquire. |
 |
|
| One thing that the "charged mercury" will not
remove is heavy oil or grease. For that you will have to use some type of detergent. When
you get some charged Mercury and put it in water you will note that it fizzes giving off
hydrogen gas (don't forget that hydrogen gas is flammable and can explode). That basically
is how "charged mercury" works. Sodium reacts with water, and when the fizzing
stops it means that all the sodium has reacted and it is now "discharged" back
to ordinary mercury. Now you must recharge it, if you want to use it as charged mercury
again. Charging mercury is such a simple procedure that it is amazing that so few prospectors know how to do it. In order to charge Mercury, you must first make concentrated solution of sodium chloride (salt) and turn your Mercury into an electrolytic cell. Mercury is poured into the bottom of a container and the salt solution is poured on top of the mercury. You should have a least a few inches of salt solution over the top of the mercury. An insulated heavy copper wire is put down into the mercury, and the insulation should go right into the mercury, so the copper is not exposed to the salt solution. A graphite rod is stuck into the solution but not touching the mercury - a good gap between them needs to be maintained. DC current from a supply such as a battery charger (turn it down to 6 V) is applied to both the mercury and the graphite rod. Polarity is important, so be sure to hook the plus and minus to the right contacts. The plus side should be attached to the carbon rod, and the minus to the wire. When you done it right, Chlorine gas bubbles off the graphite rod, and sodium goes into the mercury. This is actually a dangerous process, because chlorine is quite toxic in its own right (chlorine is outlawed under the Geneva Convention as a weapon). Chlorine gas can kill you quickly, so be very careful and don't breathe it. This is actually a miniature version of the industrial process used around the world to make chlorine gas, chlorine bleach (like we use in our washing machines) and lye. Eventually the mercury will become hardened, after which it is now fully charged and the voltage can be shut off, and the charged Mercury used. This process must be done outdoors, and you should not attempt this procedure unless you have a full understanding of the chemistry and safety hazards involved. If you don't understand the hazards and don't want to learn them, just use your Mercury and an uncharged state. Mercury is best stored in an uncharged state and then charged and used as needed. To be honest, I do not charge my mercury. |
Recommended Metal Detectors For Gold Prospecting:
|
|
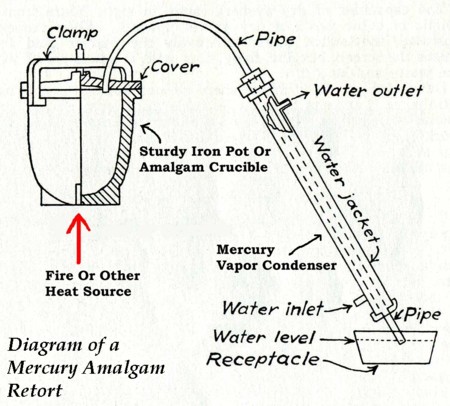
| In order to remove all the dissolved/amalgamated metals from Mercury
you will have to retort or distill it. Using Nitric acid is an alternative for small
amounts of amalgam, and I'll talk more on that in a minute. Be sure if you are using
charged mercury, that you have discharged it before you retort it. In order to distill
Mercury you should purchase a Mercury retort. They are relatively inexpensive. Yes, you
can make a retort from iron pipe fittings etc, but unless well-designed, these can be
inconvenient to use. However with a little bit of planning, design and some welding, a
decent retort can be made of pipe. There are a variety of methods used to cool the mercury
vapors coming out of the retort. There are
two types of Mercury retorts, which are vented and non-vented. The non-vented type is
simply a boiling vessel and a cooling tube from which the Mercury drips into a catch
vessel. With this type of retort NEVER
put the exit end under water in a catch vessel. If you do, a very slight drop in the
temperature of the boiling Mercury will create a vacuum sufficient to suck water back
through the system right onto the boiling Mercury. This will almost certainly create an
explosion, blowing iron shrapnel, poisonous mercury vapors and your gold all over the
place. Please believe me when I tell you,
that it is a bad idea and extremely dangerous! It may well ruin the rest of your life and
probably put you in the hospital (if you are lucky). One thing to note is that as mercury
come out of the retort and into the mercury receptacle, the water level will rise as
mercury is added to the receptacle. This needs to be noted so that you don't get in
trouble with the problem of water being sucked into the retort vessel as described above. |
 |
|
The vented type of retort is made specifically to prevent this problem. In this type, near the exit end of the retort there is a very small tube, which extends upwards a few inches. The purpose of this tube is to allow you to immerse the exit end of the retort in water. If your heat source should fail and the temperature in the "hot vessel" drop, air will be sucked in through the small tube instead of water through the exit. In any case, If you retort your Mercury you will now have Mercury, which is, for all practical purposes, clean and pure. Any amalgamated metals such as gold will be left behind in the retort. The alternative for small amounts of mercury is to use nitric acid to separate it. Gold is not soluble in nitric acid, but mercury is. Use dilute nitric acid rather than more concentrated forms. The solid material left behind after all the reaction is complete is your gold. It may be brown or dark - not the bright gold color associated with metallic gold. Like retort sponge, it can be melted down to pour a button or bar, and once melted down and poured, it will be the normal gold color. Here are a few important facts about Mercury: Mercury will react with Nitric Acid and with hot concentrated Sulfuric Acid. Mercury will not react with dilute Hydrochloric Acid, cold or dilute Sulfuric Acid, or Alkalis. Mercury will react with ammonia in the presence of oxygen. Mercury will react with and dissolve in cyanide salts in the presence of oxygen. Mercury can be recovered from solution by cementing (displacement reaction) with iron. Mercury will form amalgams with almost all metals except Iron and Aluminum. Again to sum this up, I present this information as a historical reference only. It is incomplete, and does not tell you all you need to know to safely process gold using Mercury. Personally, I strongly urge individual prospectors to use techniques other than Mercury recover the fine gold. I just find the alternatives safer and easier. I recommend further research into chemistry books, safely hazard references and mining reference texts if you want to use Mercury for yourself. |
||
Want to know a little bit more about this crazy prospector guy? Well, here's a little bit more about me, and how I got into prospecting: Chris' Prospecting Story Interested in seeing more gold? Here are some interesting photos of beautiful Gold Nuggets