| Nearly every college level
biology text book starts with the work of
Stanley Miller as the cornerstone of scientific evidence supporting the natural origin of
life. In 1953, a brilliant young graduate student named Stanley Miller, published a report
that forever changed the field of life origin studies. Miller demonstrated how an electric
spark (simulating lightening) passed through simple inorganic gasses said to resemble the
conditions of the early earth, resulted in the formation of amino acids, the basic
building blocks of most living tissues. Also in 1953, Watson and Crick published their now
famous work on the double helix structure of DNA, demonstrating how basic chemical processes convey the
traits of the parents to their offspring. Given the tremendous impact of those
two discoveries, some proposed that we had nearly completed a demonstration showing how the
first life formed in early oceans from the spontaneous assembly of non-living materials,
and how, through the processes of evolution, the genetic materials of life organized
themselves from the simplest primordial forms to modern man. These thoughts were
summarized in the popular press as the "molecules to man" theory.
Indeed, many scientists rushed in to build on Miller's initial work, and
demonstrated that, using various input gas modifications and powered with
heat, light, electric sparks or shock waves, at least trace amounts of
nearly all 20 amino acids used in life proteins could be produced in setups
similar to those used by Miller. To many it really did seem as if the
age-old question as to how life originated had finally been completely
answered. |
 |
|
|
However on further
consideration, many of the initial conclusions began to fall into doubt. Most
geo-scientists have now concluded the earth never possessed an atmosphere that was similar
to that used by Miller in his experiments. When an atmosphere similar to
what actually existed on the early earth was used, no useful amino acids
were found in the result. Although all introductory biology textbooks
still tout the Miller scenario as fact, many scientists now believe that few, if any,
amino acids were formed by the methods he identified. A more recent
hypothesis is that any amino acids which were
available to form the first life must have come from meteors. While a few meteors
are known to contain up to
100 PPM of organics similar to those produced by Miller, it is only a very ,
very small percentage.
It has also discovered that the
polymerization of amino acids into useful proteins and other structures in
most cases is strongly
inhibited by water - a difficult proposition when it was postulated that life evolved in
an early ocean, often famously called the "primordial soup". It was also found that the
polymerization reactions need significant energy input to proceed. This led researchers to
propose that amino acids from the "primordial soup" were washed up onto the edge
of some ancient volcano where they could be concentrated, dried and heated. Unfortunately,
when scientists tried simple drying and heating of amino acid mixtures, no useful proteins
or essential life products were formed (the "proteinoids" of Fox, et al., not
withstanding). Since 1953, scientists have discovered that the proteins of life are
extremely complex folded 3 dimensional structures, whose twisted shapes have much to do
with the ways they function. Assembling them in the proper manner so that
they do eventually fold into the correct useful shape is extremely
challenging to the most knowledgeable biochemist armed with powerful
computers. If it is no difficult for proteins to self assemble into useful
protein molecules, the chances for spontaneous production of some as yet
unknown self replicating protein suitable for nomination as the basis of
life is too far fetched for even the most faithful to believe. |
|
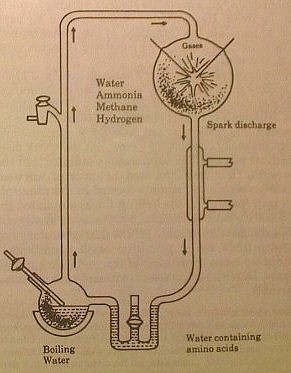
The Miller
organic synthesis apparatus.
The gasses ammonia, methane, hydrogen are
circulated past an electric arc with steam vapor from boiling water. The materials coming
out of the arc chamber are quickly cooled and contained in a special trap to keep them
further reactions. The arc is operated continually for several days, after which it is
turned off and the materials in the trap are found to contain amino acids and other
organics. |
|
|
Although much fame, honor and wealth awaits the scientist that can identify the processes how, in
a fully natural setting similar to those of the early earth, useful proteins or
self-reproductive RNA strands can self-organize and form, no one has been able to claim
the prize. In fact, nearly everything we have learned in the 50+ years since Miller first
published his work, implies that it would be virtually impossible for useful proteins or
RNA to form under fully natural, random conditions where other naturally occurring chemicals
inhibit and interfere with their formation and, even if such polymerization occurred, the
randomized mixtures would have only the most remote chance of forming a useful product.
The problems in fleshing out such a natural process are enormous, and
especially true considering the conditions likely to have existed on the
early earth. Yet, even in this,
I have not addressed the difficulties in crossing the vast chasm of organization
separating basic proteins or RNA strands and the simplest forms of life. |