
.

|
|
 |
||
|
. |
Interested in The processes of smelting or roasting your ore to recover its gold and silver? Some fairly rich ores require a lot of work to get all the gold and silver out them. Historically, smelting and roasting of ores have been used to capture the gold from difficult to treat ores. Just like those old time miners, we don't want to throw any of the silver or gold away as it can be valuable. Treating high sulfide ore is a difficult proposition, and in the article below I'll take a look at all of the best known possibilities for getting the gold and other valuable minerals out of those difficult, rebellious ores and producing a clean, saleable bullion product. Here are some things you need to think about...... |
 |
|
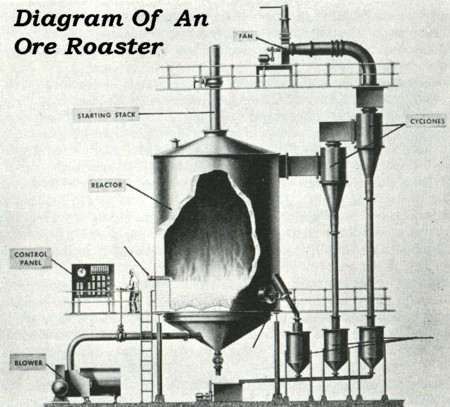
| Some prospectors are interested in the methods of processing sulfide ores to extract their values. The two of the best known processes are smelting and roasting. The two are actually different methods, but both involve heating the ore to high temperatures. Technically, any methods which consist of processing ore by heating it are called “pyrometallurgy”. | ||
| The old timers took advantage of the chemical instability of sulfides
by simply heating (or roasting) them to burn off the sulfur. Almost all sulfides will
oxidize quickly when they are heated to a high enough temperature. The
problems with processing sulfide ores by the normal methods and the fact that most of those problems can be
removed by first roasting the ore, led to the development of a variety of ore roasting systems that were
installed in the mills of that day. Heating ores to elevated temperatures is called
roasting, and it causes the oxygen in the air to convert the sulfur in the ore to sulfur
dioxide, which is a gas and is emitted to the air. The process works best when the ore is
crushed to small pieces before roasting. Additives,
such as common rock salt are often added to the mix before it is heated to enhance the
reaction of the sulfides. Once the ores are fully roasted, and the sulfur is driven off,
the extraction of the gold or silver from the ore is not especially difficult. Treatment
of the roasted ores was done by normal methods such as amalgamation or cyanide treatment. |
 |
|
| Unfortunately, sulfur oxides
are also pretty nasty and smelly air pollutants – which is why there are very few
roaster or smelters still operating and doing this type of process in the US. However,
this “burning” or roasting of the sulfide ores does work well to convert
rebellious ores into a more free milling state, allowing the values to be extracted. Exact
temperatures used in roasting vary, and excessive temperatures can cause problems,
including melting the sulfides before they can oxidize. |
||
Over the years, a number of small scale miners have sought to treat small lots of ore by burning off the sulfur as sulfur dioxide in the open air. Unfortunately, sulfur dioxide is rather pungent and offensive stuff. It's a serious air pollutant it can be toxic if you get a real heavy wiff. If you start choking and coughing, that's a real good sign you're getting too much. As if that's not enough, any arsenic in the ore will be vaporized and emitted as poisonous arsenic trioxide – and those are some very toxic air pollution fumes. This is a concern because Arsenic is actually very common in many gold ores. Selenium, mercury and other toxic elements are also found in many high sulfide gold and silver ores as well. To roast sulfide ores, it takes a good red heat and a good amount of air, but not too hot as you don't want to melt them. As previously noted, materials that have been crushed to small sizes reacts better with the oxygen in the air. Don’t seal up the material, as it needs to have access to air for the oxygen to react with the sulfur. Even if you get everything right and you're successful, the stink will probably cause the neighbors to call the police on you! That's why I can't recommend “at home” roasting methods to small miners - I think you would find it a potentially dangerous process. |
 |
|
One of the
unusual things about sulfides is that when heated to high temperatures they will melt. The
product of melted sulfides is called a matte, and is highly corrosive liquid. Converting
that molten sulfide matte to metal is called smelting. Small scale smelting can be done in
a crucible – in fact that is what a fire assay is. When smelting assays are done on
high sulfide ores, a couple of iron nails are added to the mix. The metal in the nails
reacts with the metals in the sulfides reducing the metals to their metallic state. This
is handy when processing silver containing minerals to convert them to silver metal. The
addition of scrap iron to smelt mixes is a useful technique to small-scale operators
experimenting with smelting techniques on gold-silver ores. It could also be used with
small lots of sulfide concentrates. |
||
| On the other hand, there are alternatives for processing sulfide
ores. The way the Spanish did it, the old “patio” process - is still a
possibility for small miners. Actually more recently the big mining companies have come
around to adopting a modernized use of this ancient practice, because it's less polluting. They do their treatment in a pressurized container called
an autoclave, which greatly speeds up the oxygen treatment process. For small scale treatment by individuals, the method is to grind the ore up finely, put it in some sort of tub and keep it moist. Stir and turn it over from time to time to allow the air access to the material. Natural bacteria found in sulfide ore will do the rest. Add a tiny bit of lawn fertilizer to the water you mix in to help the bacteria along. Don’t submerge the ore, just keep it wetted down and moist. In this kind of environment, the natural bacteria will break down the sulfides and convert them, using the oxygen in the air, to sulfates. It's slow and may take months to complete, and you will se the formation of salt crusts as it works. There may also be a little bit of stink, but nothing like roasting. Even in spite of this, your oxidized sulfide solutions will be strongly acidic and may be full of toxic heavy metals like lead, antimony, cadmium and copper. You may need to neutralize them, and it is also possible that they may well legally constitute a hazardous waste, so you can’t just toss them in the trash or dump them in your yard. As a result, any treatment of sulfide ores needs to be done with an eye toward compliance and any applicable environmental regulations. If you want to try something like this, be sure to do some through research beforehand. |
 |
|
Want to know a little bit more about this crazy prospector guy? Well, here's a little bit more about me, and how I got into prospecting: Chris' Prospecting Story Interested in seeing more gold? Here are some interesting photos of beautiful Gold Nuggets