|
.
Stilbite Mineral Facts:
Chemical Formula: (Na2,Ca)Al2Si6O16.6H20
The
hydrated silicate of aluminum, sodium, and calcium.
Colors:
white, sometimes pink to red,
yellow, or brown.
Streak
nearly colorless.
Hardness:
3 to 4
Density: 2.2
Cleavage:
Perfect cleavage on
(010)
parallel to clinopinacoid and imperfect parallel to (001).
Crystallography: Monoclinic
Crystals
uniformly found in the form of cruciform twins usually thin and tabular.
Commonly tabular parallel to clinopinacoid. Crystals usually in
sheaflike aggregates or twinned crystals with an orthorhombic habit
resembling the simple twins of phillipsite.
Luster:.
Vitreous luster that is
nearly pearly on
the cleavage planes. It is transparent to slightly translucent.
|
Stilbite Crystals, India |
|
Composition,
Structure and Associated Minerals:
Stilbite occurs in the vacuoles
of amygdaloidal basalts,
in veins cutting granites and other coarse-grained rocks, and on the walls
of cracks in gneisses and schists. It occurs also as deposits around hot
springs. Typically associated with a variety of other zeolite minerals like
heulandite and laumontite.
Stilbite is also a zeolite itself. It is especially common filling the steam
cavities of lavas, as in the basalts. It is a mineral of secondary origin
found in amygdaloidal cavities in basalts and related rocks. It is found
associated with various zeolites, as well as
calcite,
phrenite and
quartz.
Zeolites are a class of hydrated silicates of aluminum with sodium and
calcium as the important bases. The name stilbite is derived from a Greek
word meaning luster. Stilbite is known as desmine in some countries.
|

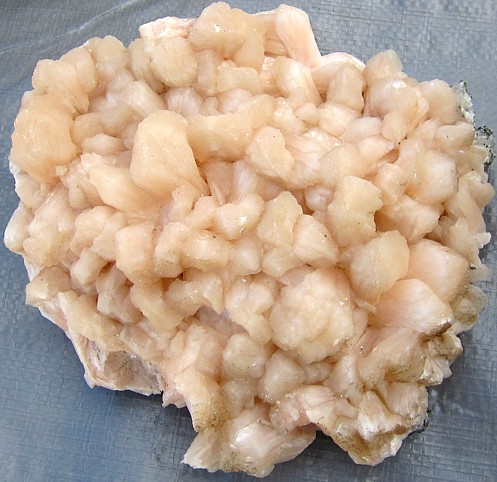
Stilbite (pink) |
|
|
Identification
and Diagnostics
Before the blowpipe stilbite
exfoliates, swells and crinkles to a white blebby glass, fusing with
intumescence. In the closed tube it yields water and becomes cloudy and
opaque. It is decomposed by hydrochloric acid with separation of silica but
without the formation of a jelly, producing a pulverulent silica. Its powder
reacts alkaline. Characterized chiefly by its cleavage, pearly luster on the
cleavage face, associated minerals and common sheaf like groups of
crystals.
Occurrence,
Localities and Origins:
Its principal localities are in
the cavities of basalt rocks of places like the Isle of Skye, Arran in
Scotland; Mourne Mts. and the Giant's Causeway, in Ireland; and the Deccan
trap volcanic rocks of India. It occurs in veins at Radauthal in the Harz;
at Striegau, in Silesia; and at Falun, in Sweden. It is abundant in the old
volcanic rocks of Nova Scotia; around Lake Superior, and of Table Mt., near
Golden, Colo., and near Bergen Hill, N. J., and is present in cavities in
gneisses at several points in Connecticut and Pennsylvania. Notable
localities for its occurrence also include Poonah, India; the Faroer
Islands; Kilpatrick, Scotland; Iceland and Nova Scotia. The tertiary
Deccan basalts of western India are the most important sources of stilbite
in the world. Stilbite is also the most abundant zeolite in the tholeiitic
basalt plateaux near Nasik and Pune in Africa.
Uses:
Silbite is used in crude oil processing as the open channels in
the stilbite crystal structure act like a molecular sieve, enabling it to
separate various hydrocarbons produced in the process of petroleum refining.
Fine, well formed crystals are also appreciated as mineral specimens by
collectors around the world.
Return to the
Mineral Collectors Information Page
|


