|
.
Zeolite Mineral Facts:
Chemical Formula:
Variable for the group
The group known as the
zeolites comprises minerals that are hydrous silicates of aluminium with
calcium, sodium, potassium, barium or strontium. The calcium compounds
are commonest, followed by the sodium compounds. Compounds with the
other elements are comparatively rare.
Colors:
Various mostly light colored.
White in color when free from iron
and other impurities.
Hardness:
ranging 3.5 to 5.5
Density: 2
to 2.4
Cleavage:
Most zeolites have
one or more cleavages planes.
Crystallography: many are Monoclinic
Other crystal forms are also present in
zeolites including
orthorhombic, hexagonal, isometric, etc.
Luster:.
Vitreous
luster. Normally translucent to transparent.
Optics:
(Refractive Index):
= variable
|
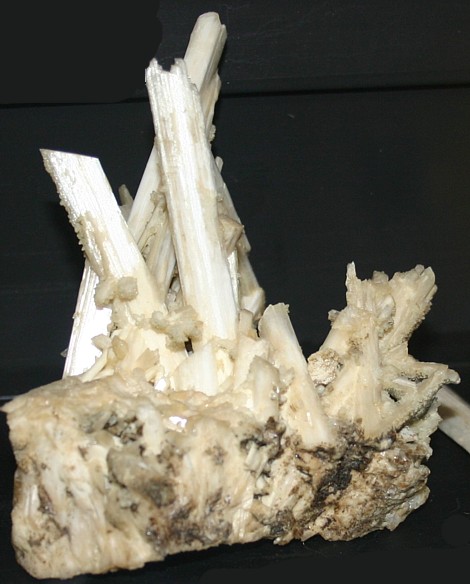
Zeolite Laumanite |
|
Composition,
Structure and Associated Minerals:
The zeolites show close
similarities in composition and in their associations and mode of
occurrence. Naturally occurring zeolite deposits are rarely pure and
are normally contaminated to varying degrees by other minerals.
Zeolites are commonly associated with minerals like
calcite, pectolite, datolite,
prehnite,
quartz and other zeolites. For this
reason, naturally occurring zeolites are excluded from many important
commercial applications where uniformity and purity are essential. In those
cases where purity is required, synthetic zeolites are used. Over 40
naturally occurring zeolite mineral framework structures are known.
Identification and Diagnostics
Before the blowpipe all the zeolites fuse with intumescence, or bubbling,
and all give water in the closed tube. Most can be attacked and broken down
by hydrochloric acid. They are comparatively soft (3.5-5.5), and have a low
specific gravity (2-2.4). The most common natural zeolites are:
Ptilolite, Hetdandite, Phillipsite, Harmotome,
Stilbite, Laumontite, Scolecite, Natrolite, Thomsonite, Chabazite and
Analcite.
Occurrence,
Localities and Origins:
Natural zeolites form where volcanic
rocks and ash layers react with alkaline groundwater of either salt or fresh
water origins. Zeolites are secondary products derived by the alteration and
hydration of alkali aluminum silicates, such as the feldspars, leucite,
nepheline, etc. Zeolites then crystallize in secondary post depositional
environments over periods ranging from thousands to millions of years in
shallow marine basins. They are nearly always found in veins, or on the
walls of crevices in rocks which are most often volcanic rocks, where they
have been deposited by the circulating waters. All are well crystallized and
some of them are formed into complicated crystals and make attractive
mineral specimens.
|

Above: Zeolite Chabazite with Apophyllite |
|
|
Notable localities for
stilbite include Poonah, India; Isle of Skye; Faroer Islands; Kilpatrick,
Scotland; Iceland; Nova Scotia. Notable localities for chabazite are the
Faroer Islands; Greenland and Iceland; the Giant's Causeway, Ireland; at
Aussig, Bohemia; in Nova Scotia, etc. Fine crystals of analcite are found at
Bergen Hill, New Jersey; in the Lake Superior in the
copper ore district; at Table
Mountain, near Golden, Colorado; at Cape Blomidon, Nova Scotia; in the
Cyclopean Islands near Sicily; in the Fassathal, Tyrol; on the Faroer
Islands; in Iceland. Notable localities for the occurrence of natrolite are
Aussig and Teplitz, Bohemia; Puy de Dome, France; Fassathal, Tyrol; Kapnik,
Hungary; in various places in Nova Scotia; Bergen Hill, New Jersey; copper
district, Lake Superior.
USES:
Zeolites are widely used in industry
for water purification, as catalysts, for the preparation of advanced
materials and in nuclear reprocessing. Some are used in the refining of
petroleum. Their biggest use is in the production of laundry
detergents. They are also used in medicine and in agriculture..
Return to the
Mineral Collectors Information Page
|

Zeolite Natrolite
|



